Understanding the role of eye pressure in glaucoma
Consistent eye pressure control is important.
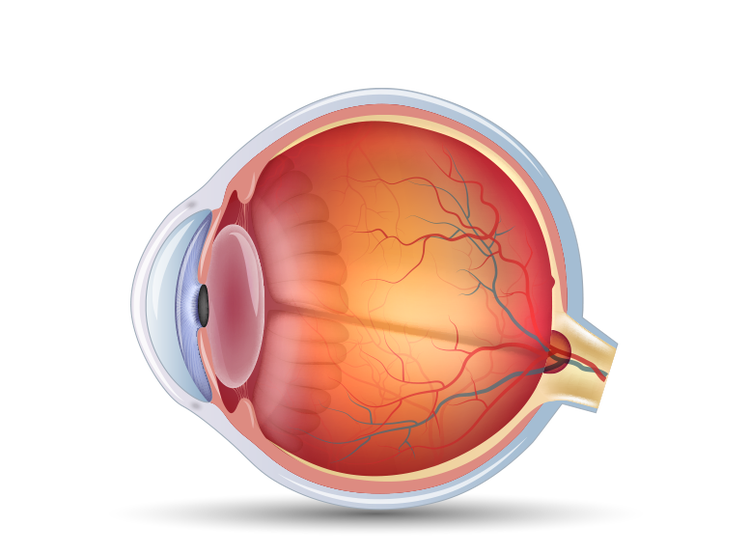
The pressure inside your eye is called intraocular pressure, or IOP. When IOP is elevated, it's a major risk factor for vision loss associated with glaucoma over time. The higher the level of IOP, the greater the chance of optic nerve damage and vision loss.
1
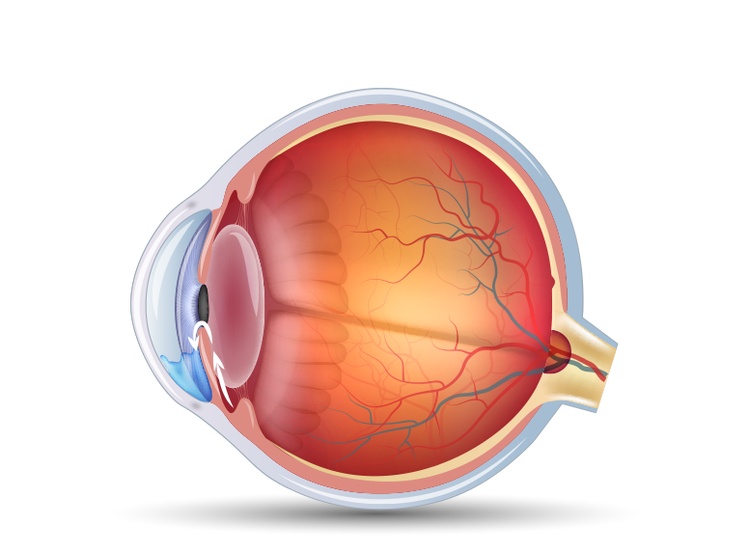
Fluid builds up inside your eye and pressure increases
2
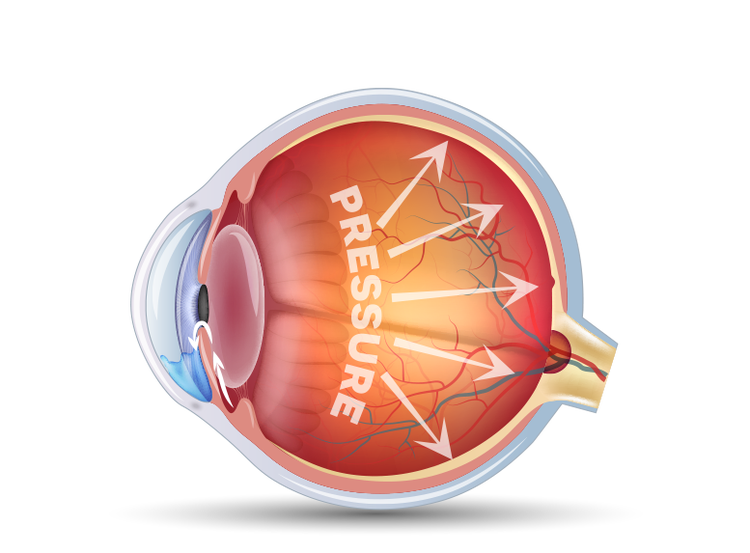
As pressure increases, it can damage your optic nerve (which connects your eye to your brain)
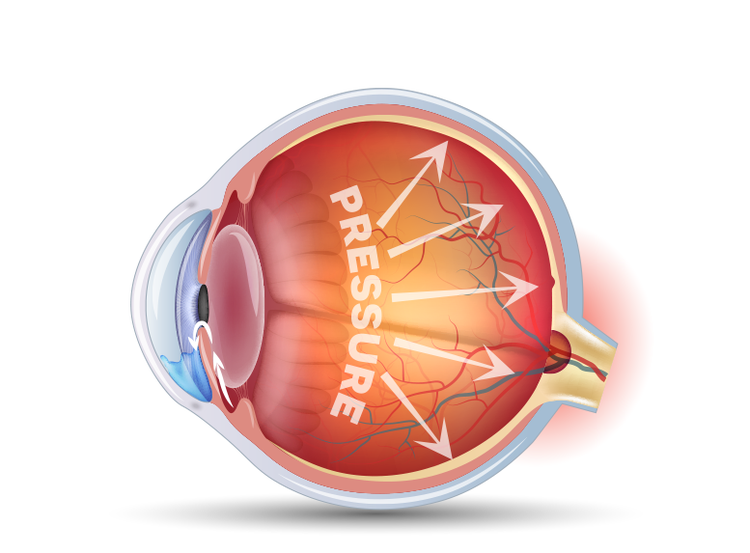
3
Over time, this damage to the optic nerve can lead to vision loss
GLAUCOMA VISION
LOSS SIMULATOR
This simulator shows you how elevated eye
pressure may affect your vision over time



Divider
Just 1 DURYSTA® implant works for several months
DURYSTA® is a tiny, dissolvable implant your eye doctor places in your eye
- The first and only FDA-approved dissolvable ocular implant to reduce eye pressure in people with open angle glaucoma or high eye pressure (ocular hypertension)
- Just 1 DURYSTA® implant reduced eye pressure for 15 weeks in clinical studies
- As DURYSTA® dissolves, it automatically releases medicine to help reduce high pressure inside your eye
DURYSTA® should not be given in the same eye more than once.
The most common side effect involving the eyes reported in patients using DURYSTA® was eye redness. Other common side effects reported were: feeling like something is in your eye, eye pain, being sensitive to light, a blood spot on the white of your eye, dry eye, eye irritation, increased eye pressure, a loss of cells on the inner layer of the cornea, blurry vision, inflammation of the iris, and headache.
Divider
Meet Monica, a real glaucoma patient
Divider
Resources to help on your own DURYSTA® journey

https://www.durysta.com/pdfs/durysta-patient-in-office-screener.pdf
Find out if DURYSTA® is right for
you with this checklist.
Download it now and get
the conversation started.

https://www.durysta.com/pdfs/durysta-patient-savings-program-flashcard.pdf
Find out how the Savings
Program could benefit you.
Download the Savings Program
Flashcard to learn more.
Frequently Asked Questions About DURYSTA®
DURYSTA® is placed directly inside your eye, 1 time only per eye, by your eye doctor.
DURYSTA® should not be given in the same eye more than once.
The most common side effect involving the eyes reported in patients using DURYSTA® was eye redness. Other common side effects reported were: feeling like something is in your eye, eye pain, being sensitive to light, a blood spot on the white of your eye, dry eye, eye irritation, increased eye pressure, a loss of cells on the inner layer of the cornea, blurry vision, inflammation of the iris, and headache.
See full Prescribing Information for complete safety information.
One DURYSTA® implant lasts for several months.


Yes! Ask your doctor about the DURYSTA® Savings Program—eligible commercially-insured patients pay $0* for up to one implant per eye
*Offer valid only for commercially-insured patients with plans covering DURYSTA; patient out-of-pocket expense may vary. Offer not valid for patients receiving reimbursement from Medicare, Medicaid, or any other federal, state, or government-funded healthcare program. See Program Terms, Conditions, and Eligibility Criteria here or visit www.durystasavingsprogram.com.
DURYSTA® is tiny.
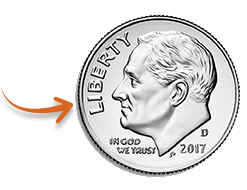

It's smaller than the “I” in LIBERTY on a dime!
Dime enlarged to show detail.
Divider
DURYSTA® may be right for you, too
Ask your eye doctor about DURYSTA®
Find out if DURYSTA® is right for you.
Here are some things to think about before speaking to your eye doctor:
- Is elevated eye pressure an issue?
- Do you understand elevated eye pressure and the risks associated with it?
- Do you have difficulty applying your eye drops every day? (Hard time using them, or forgetting?)

Find an eye doctor near you who can prescribe DURYSTA®


Ask your doctor about the DURYSTA® Savings Program—eligible commercially-insured patients pay $0* for up to one implant per eye
*Offer valid only for commercially-insured patients with plans covering DURYSTA; patient out-of-pocket expense may vary. Offer not valid for patients receiving reimbursement from Medicare, Medicaid, or any other federal, state, or government-funded healthcare program. See Program Terms, Conditions, and Eligibility Criteria here or visit www.durystasavingsprogram.com.
Program Terms, Conditions, and Eligibility Criteria:
1. This offer is valid only for commercially-insured patients 18 years of age or older whose insurance plans cover DURYSTA® (bimatoprost intracameral implant) 10 mcg. 2. This offer is not valid for use by patients receiving reimbursement under any federal, state, or government-funded healthcare programs (e.g., Medicare, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs); private indemnity or HMO insurance plans that reimburse patients for the entire cost of their prescription drugs; or where prohibited by the patient’s health insurance provider. If at any time a patient begins receiving prescription drug coverage under any federal, state, or government-funded healthcare program, patient will no longer be eligible for this offer and must call IQVIA Inc. at 1-833-DURYSTA, option 2, to stop program participation. This offer is not valid for cash-paying patients. This offer is also not valid for commercially-insured patients whose insurance plans do not cover DURYSTA. 3. Depending on insurance coverage, eligible commercially-insured patients pay $0 for each of up to one (1) DURYSTA implant per eye. This offer applies to the implant only and does not apply to costs for any other medication, procedure, or diagnostic service. Check with healthcare provider and insurance plan for discount. Patient out-of-pocket expense may vary. 4. Primary payer benefits must be applied prior to submitting a savings request on behalf of a patient. Patients may not seek reimbursement for value received from the DURYSTA Savings Program from any third-party payers. 5. Offer applies to implants administered during the current program period of July 1, 2022 through June 30, 2023. Savings requests, including the DURYSTA Savings Program Physician Reimbursement Request Form, the CMS-1500 claim form, and the Explanation of Benefits (EOB) from the primary payer, must be submitted online at AllerganEyeCue.com or by fax to 1-866-676-4069 within 180 days after the product is administered to the patient. 6. Allergan, an AbbVie company, reserves the right to rescind, revoke, or amend this offer without notice. 7. Offer good only in the USA, including Puerto Rico. Patients residing in or receiving treatment in certain states may not be eligible to participate in this program. 8. Void if prohibited by law, taxed, or restricted. 9. This offer is not transferable. The selling, purchasing, trading, or counterfeiting of this offer is prohibited by law. 10. This offer has no cash value and may not be used in combination with any other discount, coupon, rebate, free trial, or similar offer for the specified prescription. 11. This offer is not health insurance. 12. Program expires June 30, 2023. 13. B y redeeming this offer, patient represents they meet the eligibility criteria above and patient understands and agrees to comply with the terms and conditions of this offer.
For questions about the program, please call 1-833-DURYSTA, option 2, or email [email protected].
Program managed by IQVIA Inc. on behalf of Allergan, an AbbVie company.












